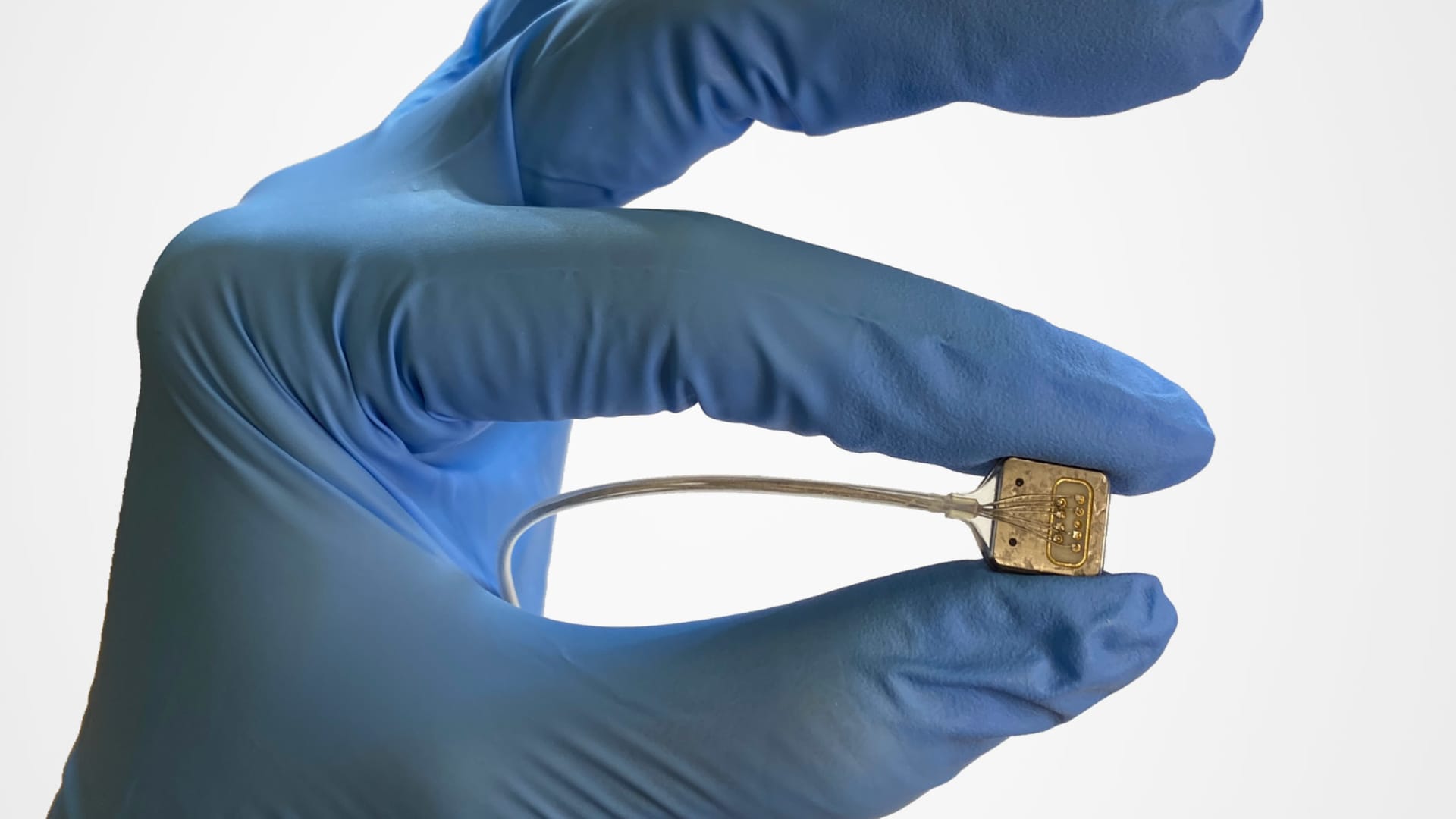
Paradromics Cortical Module
Source: Padromics
A growing team of nearly 50 employees at the neurotech startup Paradromics is working on a brain implant that sounds like the work of science fiction. And it has caught the attention of federal regulators.
Paradromics, founded in 2015, is developing a device that could help patients with severe paralysis regain their ability to communicate by deciphering their neural signals. And on Thursday, the Austin, Texas-based company announced that it has received the Breakthrough Device designation from the Food and Drug Administration for its flagship system, called the Connexus Direct Data Interface.
CEO Matt Angle said the designation, in addition to a $33 million funding round the company also announced Thursday, will help Paradromics bring its device to market.
Paradromics is part of the emerging brain-computer interface, or BCI, industry. A BCI is a system that deciphers brain signals and translates them into commands for external technologies. Experts believe the systems could someday help treat maladies like blindness and mental illness.
Perhaps the best-known name in the space is Neuralink, thanks to the high profile of its co-founder Elon Musk, who is also the CEO of Tesla, SpaceX and Twitter.
Scientists have been studying BCI technology for decades, and several companies have developed promising systems that they hope to bring to market. But receiving FDA approval for a commercial medical device is no small task — it requires companies to successfully conduct several extremely thorough rounds of testing and data safety collection.
As of May, no BCI company has managed to clinch the FDA’s final seal of approval.
Paradromics’ BCI, the Connexus Direct Data Interface, is an assistive communication device that translates neural signals into text or synthesized speech. An array of tiny electrodes is implanted directly into the brain tissue, where it measures and deciphers brain signals that are ultimately emitted to external devices through a transceiver that sits under the skin in the chest.
“It’s essentially taking some of the things that have been successful in previous clinical trials, and then improving on them from an engineering standpoint to make them better,” Angle told CNBC in an interview.
Paradromics scientists at work
Source: Paradromics
Angle said the company’s BCI is designed to last around 10 years and will initially be used to help patients who have lost their ability to physically communicate. The device will require invasive brain surgery, but Angle said the quality of the neural signals it can measure will allow patients to communicate at a faster and more natural rate than they could with a less invasive BCI, like the one being developed by Paradromics competitor Synchron.
So far, regulators seem to be on board with Paradromics’ approach. The FDA’s Breakthrough Device designation is granted to medical devices that have the potential to provide improved treatment for debilitating or life-threatening conditions.
The agency has granted 32 of these designations in fiscal 2023 so far, according to its website.
Angle said the designation will help create a “fast track” for communication between the FDA and Paradromics. It’s an advantage that could be key for getting regulators to more quickly approve future clinical trials.
The company is currently conducting animal safety trials, and the data from those trials will help the FDA determine whether to approve an in-human study. Angle said Paradromics is hoping to launch its first clinical trial with human patients in the first half of 2024.
The startup’s new $33 million funding round was led by Prime Movers Lab.
“It’s a beautiful story,” Prime Movers Lab founder and general partner Dakin Sloss told CNBC in an interview. “And it’s a real technology that’s working now, today. It’s not like a pipe dream that you gotta wait 10 years for.”
Angle said it is an exciting time in the BCI field, especially as multiple companies are working to distinguish themselves in an industry that he estimates will create billions of dollars in value. But while it is easy to get excited about the future capabilities of BCIs, Angle believes a lot of good can already be done.
“A lot of people are excited about the futuristic, kind of speculative applications. But the reality of brain-computer interfaces is, in some ways, more exciting,” he said. “It can transform what would otherwise be really challenging problems in brain health.”




